Through hands-on experiments, we'll learn about intriguing chemical reactions and how atoms combine to form different molecules.
No Sessions Available
Course Length: 4 class sessions ($16/class)
In this hands-on course, we'll explore molecules, chemical reactions and molecular behavior through fun and intriguing chemical reaction experiments! The chemical reaction experiments (using safe household ingredients) will be demonstrated by the instructor, and students are encouraged to follow along at home. (Please note: participation in the experiments is optional; younger learners may need assistance). We'll also learn how to play a fun Molecule Match card game with our Atom Element Flash Cards.
Please note: It is suggested that students have heard the basics of atoms, protons, neutrons, electrons and covalent molecular bonding prior to taking this course. If your learner has not yet learned about these topics, it is recommended to take our intro course Atoms & The Periodic Table of Elements prior to taking this course.
This 4-session course also introduces chemical equations, and reviews the underlying structural organization of the Periodic Table of Elements, particularly as it relates to electron orbital structure and molecule formation. The hands-on chemical reaction experiment will be demonstrated by the instructor, and students are invited to follow along at home if they'd like (younger learners may need assistance; participation is encouraged but totally optional). The weekly experiments include:
Class 1 - Making Carbon Dioxide Bubbles through a chemical reaction
Class 2 - Making Ice Cream with Salt, using a chemical reaction
Class 3 - Acid and Base Investigations using pH Paper: understanding acids and bases
Class 4 - Cleaning Dirty Pennies with a chemical reaction
This course includes a wide variety of sensory activities, including a hands-on chemistry demo, lecture presentation, in-class drawing activity, question-and-answer, short instructional videos and visual aids. We'll be varying activity types often to keep students engaged! This course offers engaging exploration of Chemistry and Quantum Physics concepts, providing foundational knowledge for further academic study of these subjects.
The course utilizes illustrated Atom Cards (for elements 1-54) as visual aids. Printable handouts of the Atom Cards will be provided upon enrollment for self-printing at home. Professionally-printed cards are also available.
Session Overview
Session 1:
The first class session reviews the concept of atomic structure and atoms forming molecular bonds, starting with the covalent bond and learning what drives the atoms to form this type of bond. We'll then learn new info about the various kinds of covalent bonds (single, double and triple bonds) and introduce chemical reactions and their representation using atomic symbols. We'll see a chemical reaction equation in action by doing an experiment making Carbon Dioxide bubbles.
Session 2:
The second class session expands from covalent bonds and sharing electrons, to exploring atoms donating electrons to form ionic bonds. We will learn more about the valence shell and how understanding the placement of the atoms in the Periodic Table helps us determine the kind of bonds it can form. We will identify and recognize patterns and trends in the Periodic Table to use when making molecules. We'll learn how to use our understanding of valence electrons in combination with atomic symbols to write chemical formulas and "make" new molecules on paper. Then we'll use these molecules in a chemical reaction through a hands-on demonstration of making ice cream with salt, which students can also do at home. We'll also learn the Molecule Match Card Game.
Session 3:
Session 3 further explores the trends and relationships between the atoms in the Periodic Table. We'll discuss the reasons why the atoms are arranged in this specific manner. Then we'll dive into the concept of acids and bases, and learn about the pH scale. We'll discuss how acids and bases are used for many useful things. For this week's hands-on activity, we will test different liquids to determine their pH and conduct an acid base investigation.
Session 4:
Session 4 explores more examples of chemical reactions in everyday life. We'll learn about how some metals spontaneously interact with oxygen in the air, forming a dull tarnished layer on the metal. Then we'll have a fun hands-on activity cleaning dirty pennies with a weak acid solution made of vinegar and salt. We'll discuss how the acid solution breaks apart the oxidized copper molecules, making the penny shiny again. We will also discuss the role of the neutron in an atom, and delves into atomic isotopes. We'll learn about radioactive isotopes and how they can be used in Carbon Dating to determine the age of fossils.
Explore more exciting topics below!

Students learn to use real observatory telescopes in Chile & the Canary Islands to explore a wide range of astronomy topics!

Explore exciting frontiers of quantum physics including dark matter, dark energy, antimatter and the Standard Model of Particle Physics in this engaging STEM course!
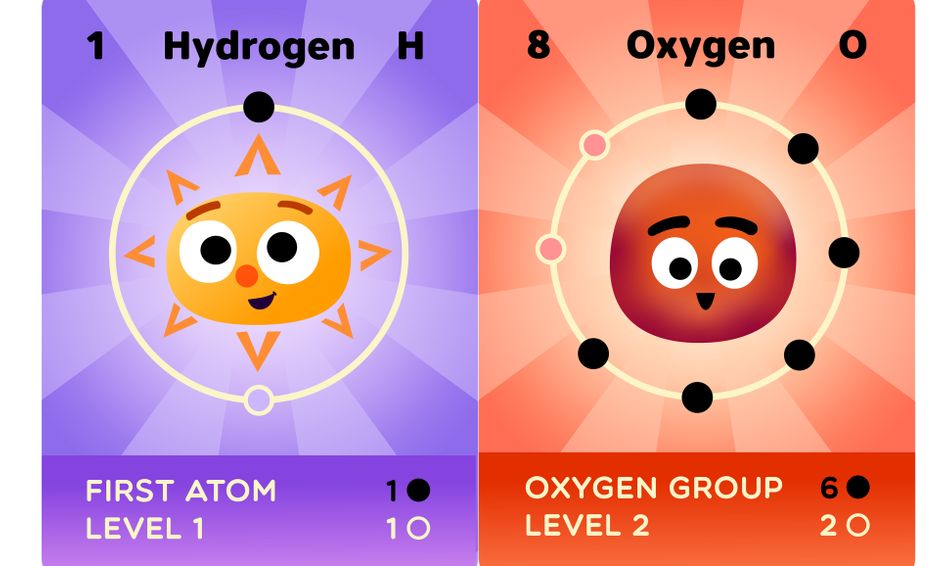
This innovative chemistry course introduces young learners to the amazing world of atoms, molecules and the Periodic Table of Elements!

In this 12-session course, we'll dive into learning about aeronautics, motion physics, airplane design, rocketry and aerodynamics!